1. What is a cold chain?
In the pharmacy world, a cold chain is a system of refrigerators, cold storage facilities, and disposable cold boxes. All of these are organized so that temperature-sensitive medications are kept at the right temperature from factory to the point of use.1
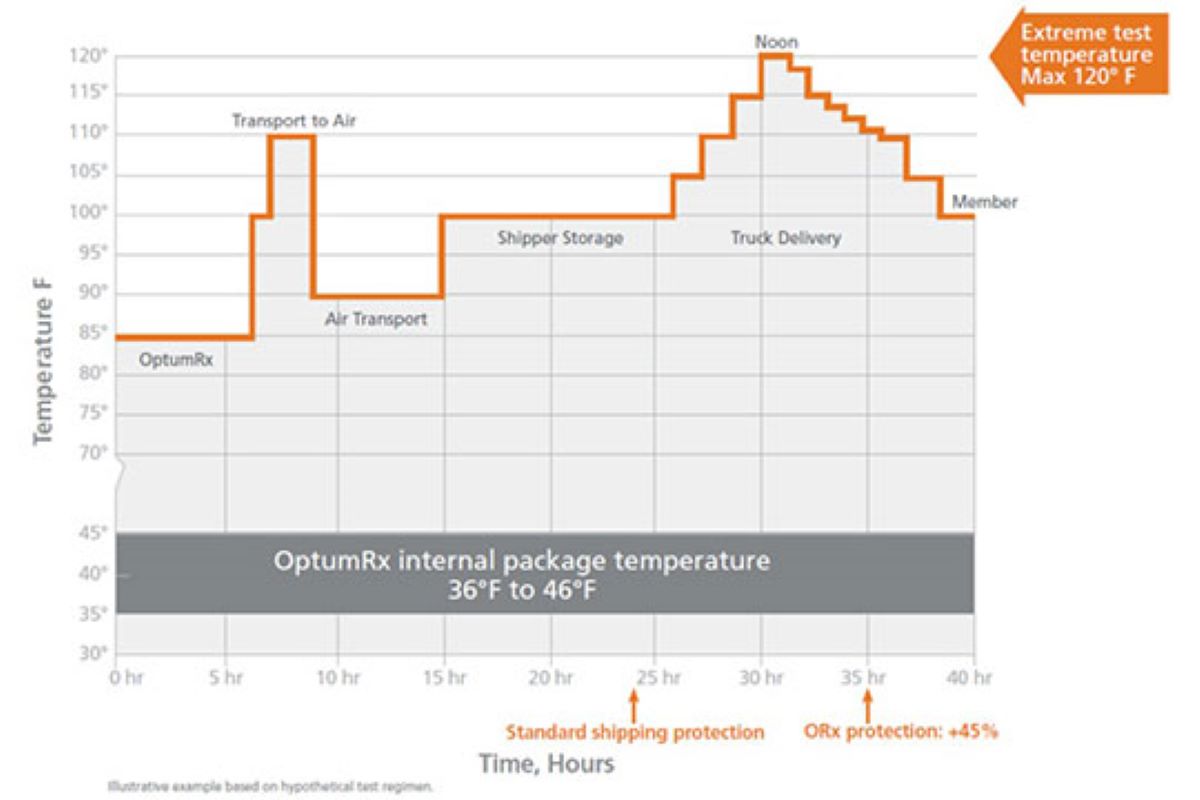
A properly functioning cold chain delivery system keeps sensitive medicines within a designated temperature range as they move through the supply system. The U.S. Food and Drug Administration requires that refrigerated biologics be stored and transported within 2°C to 8°C (36°F to 46°F) — unless a medicine is deemed stable at other temperature ranges.2
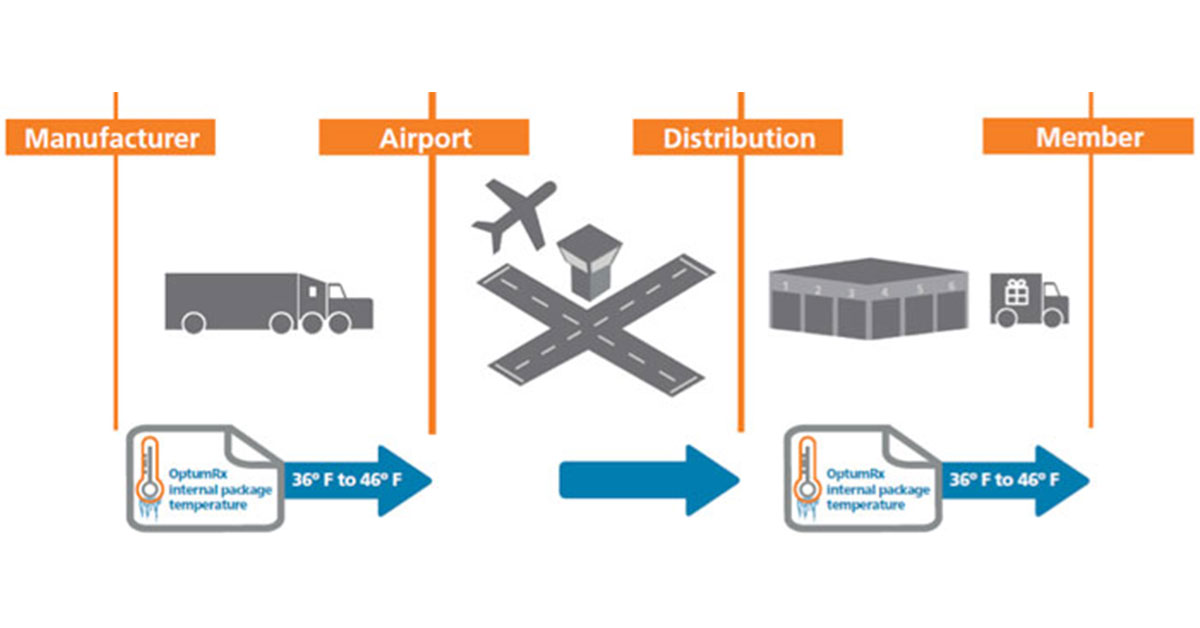
Typical cold chain distribution