Alzheimer’s disease awareness
Each September we recognize World Alzheimer’s month, with November being National Alzheimer’s Awareness Month in the U.S. Both occasions mark a time to show support for the millions of people who live with Alzheimer’s disease.1
- Alzheimer’s disease leads to the progressive loss of many essential mental and physical abilities.2
- Alzheimer’s disease is the 6th leading cause of death among U.S. adults.3
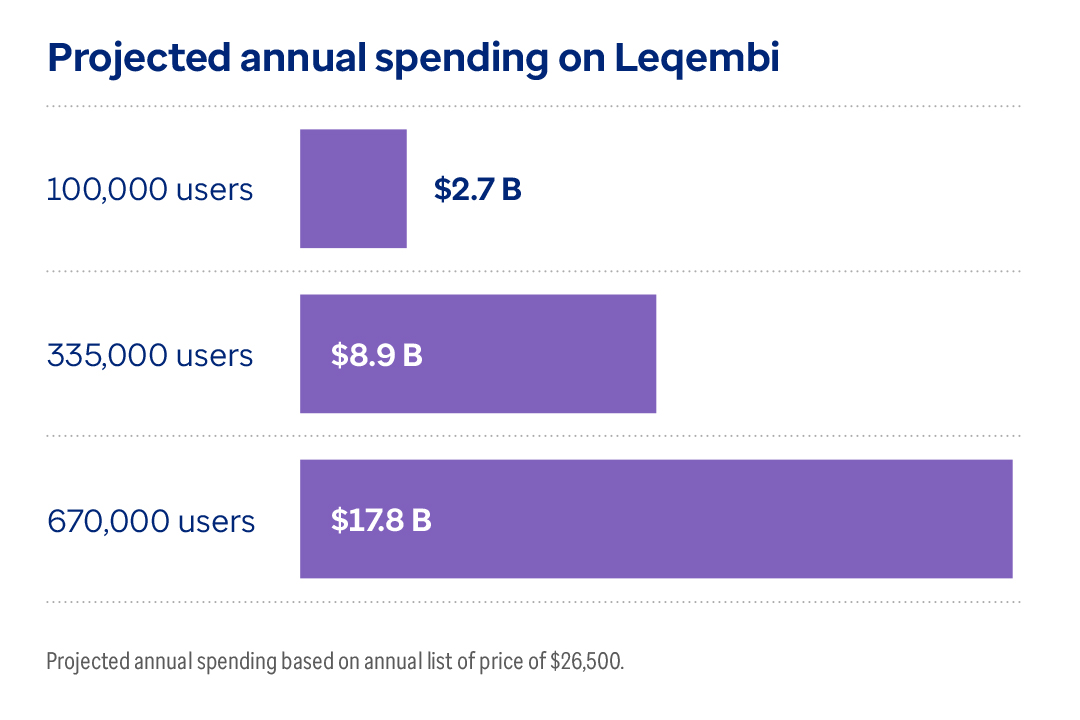
- In 2023, Alzheimer's and other dementias will cost $345 billion in the U.S.4
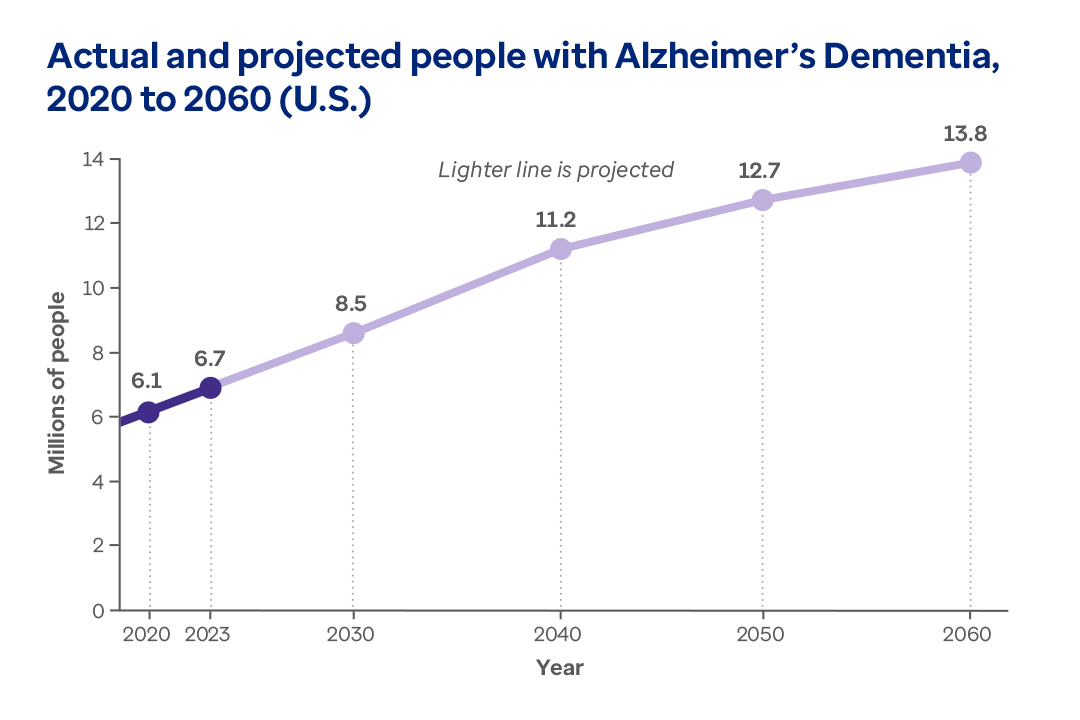
More than 6 million people are living with Alzheimer's dementia in the U.S. Up to 13.8 million may be affected by 2060.5