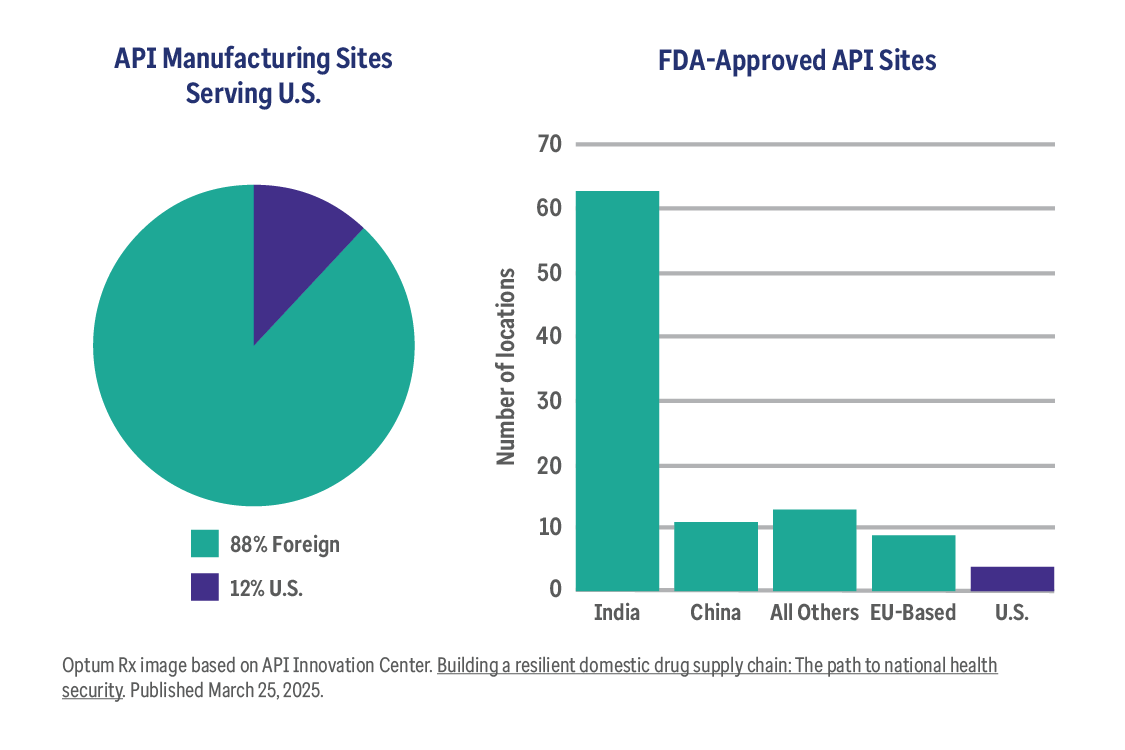
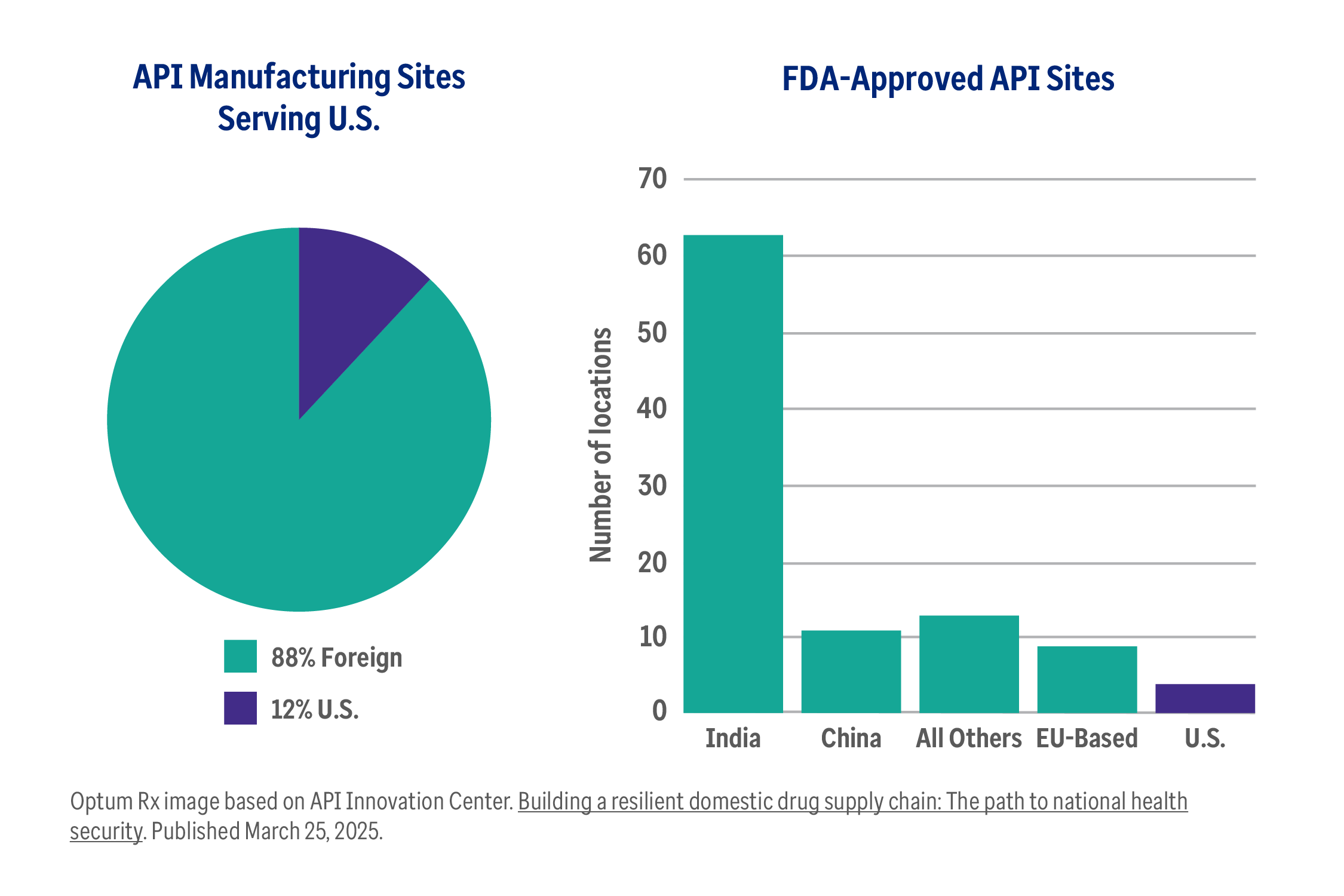
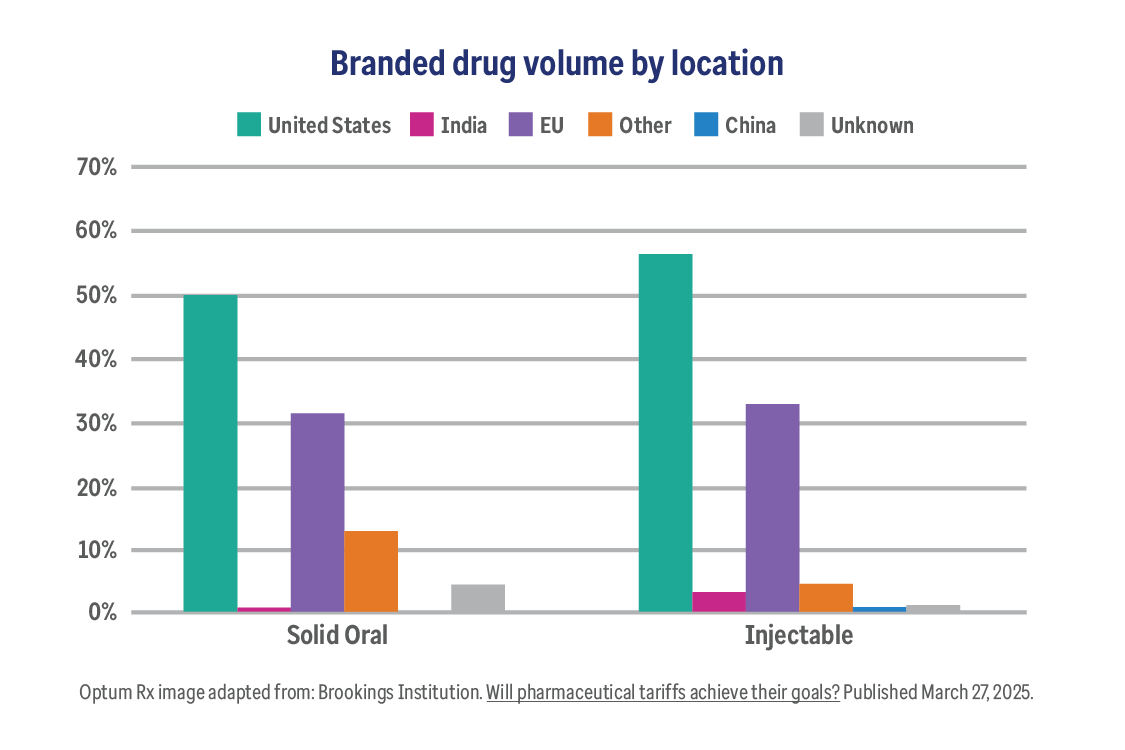
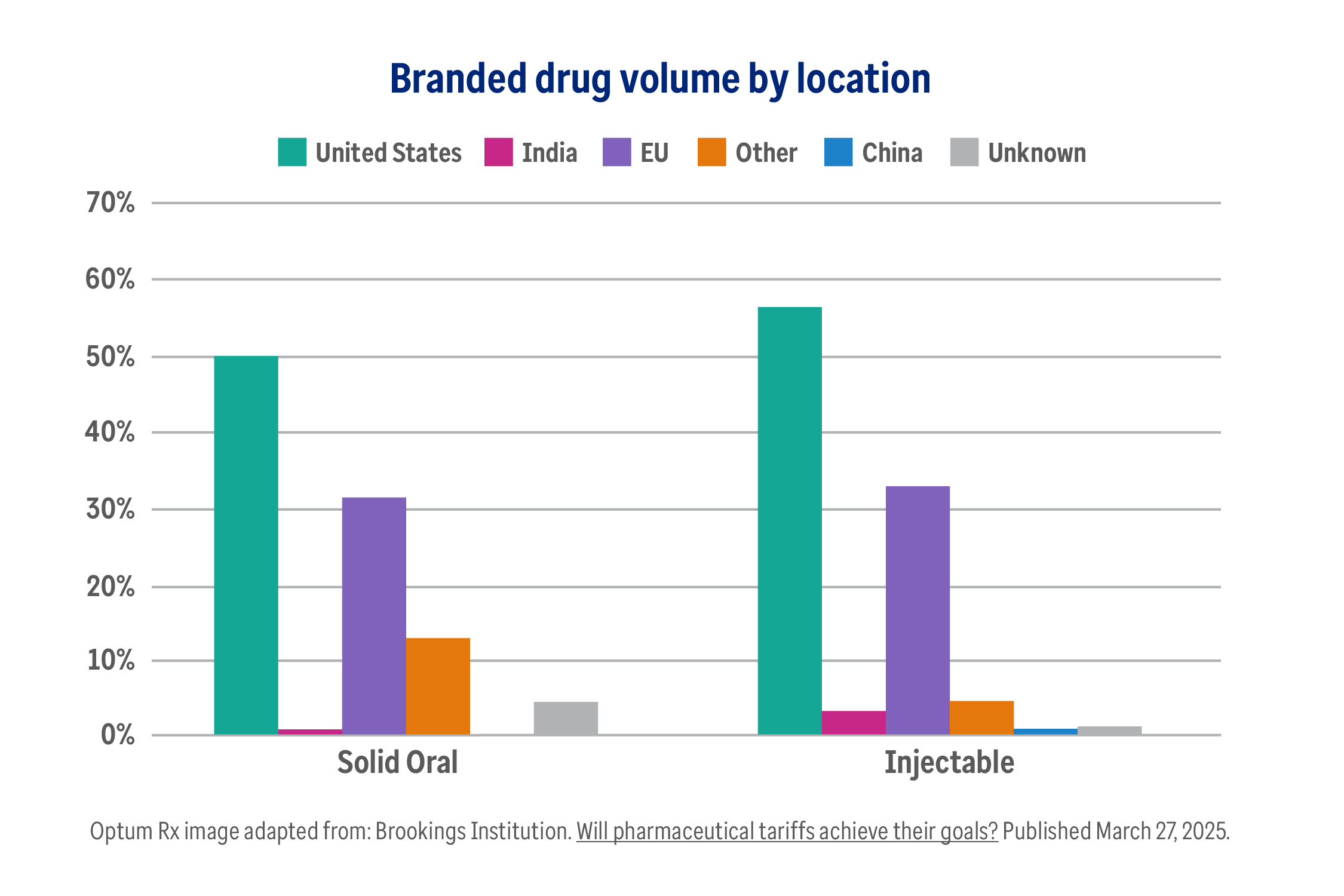
The truth about pharmaceutical tariffs
The on-again, off-again tariffs on imported goods imposed by the Trump administration are dominating the news.1 And although imported drugs have so far been excluded from new tariffs, the pharmacy world needs to pay close attention, because tariffs could be coming to imported drugs very soon.2