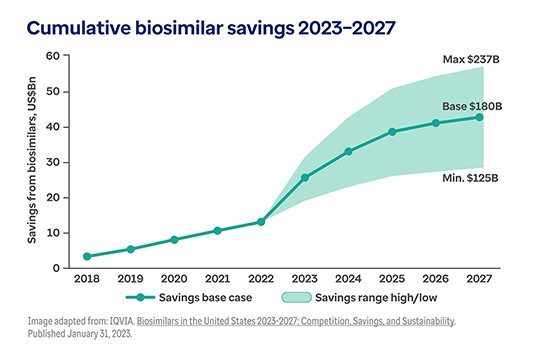
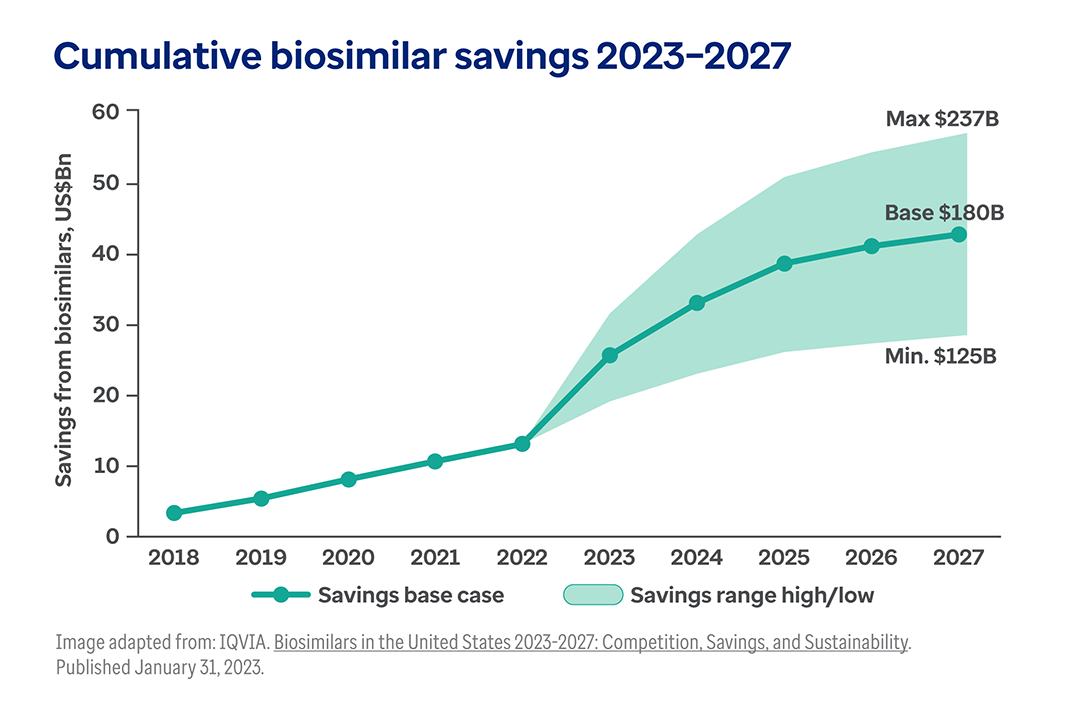
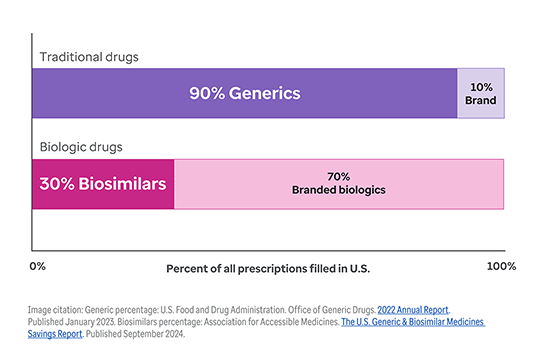
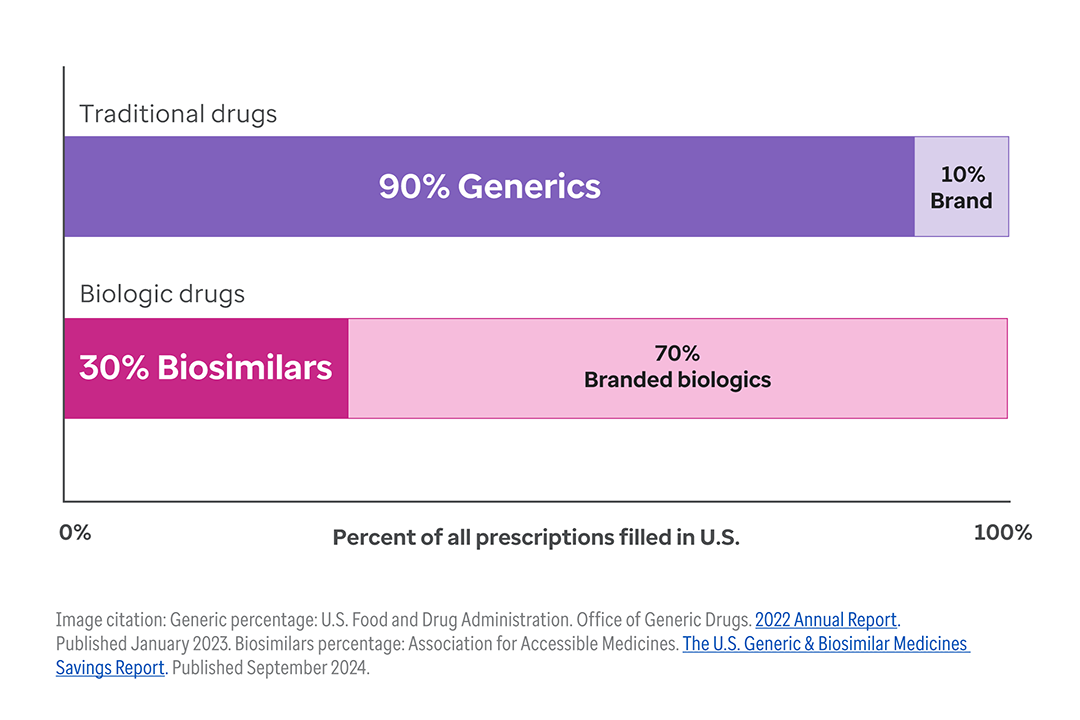
The shift toward biologic drugs is one of the biggest contributors to higher health costs.1
While only 1-2% of the U.S. population uses biologic products, they account for 46% of all national prescription drug spending.2, 3
And this trend continues to accelerate. Today, nearly half of all new drug approvals (48%) are for biologics.4